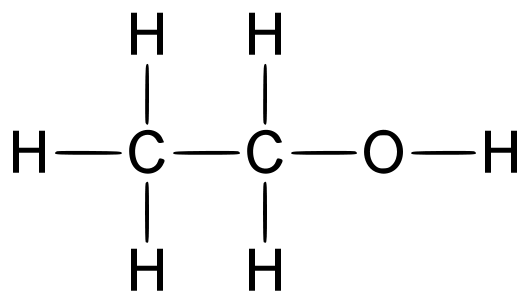
Structure of Ethanol
Ethanol molecules contain carbon, hydrogen and oxygen atoms. Ethanol can be manufactured by the hydration of ethene. In a reaction, ethene (which comes from cracking crude oil fractions) is heated with steam in the presence of a catalyst (to speed up the reaction).
Process of reaction
ThE reaction typically uses a temperature of around 300°C and a pressure of around 60–70 atmospheres. Notice that ethanol is the only product. The process is continuous – as long as ethene and steam are fed into one end of the reaction vessel, ethanol will be produced. These features make it an efficient process. However, ethene is made from crude oil, which is a non-renewable resource.
Reversing Reaction
The reaction of ethene with steam to form ethanol can be reversed. This allows ethanol to be converted into ethene. A catalyst of hot aluminium oxide is used to speed up the reaction.