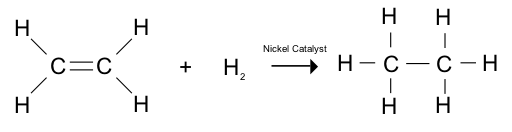
Hydrogenation
Saturated vegetable fats are solid at room temperature, and have a higher melting point than unsaturated oils. This makes them suitable for making margarine or for commercial use in the making of cakes and pastry. Unsaturated vegetable oils can be ‘hardened’ by reacting them with hydrogen, a reaction called hydrogenation.
During hydrogenation, vegetable oils are reacted with hydrogen gas at about 60°C. A nickel catalyst is used to speed up the reaction. The double bonds are converted to single bonds in the reaction. In this way, unsaturated fats can be made into saturated fats – they are hardened.
Bromine Water Test
Unsaturated vegetable oils contain carbon-carbon double bonds. They can be detected using bromine water, just as alkenes can be detected in this way. Bromine water becomes colourless when shaken with an unsaturated vegetable oil, but it stays orange-brown when shaken with a saturated vegetable fat. Bromine water can also be used to determine the level of saturation of a vegetable oil.