Esters
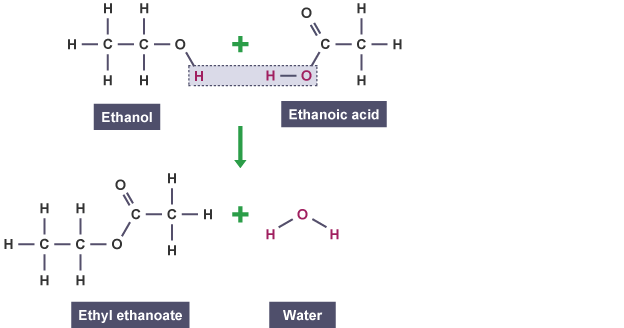
Esters occur naturally - often as fats and oils - but they can be made in the laboratory by reacting an alcohol with an organic acid. A little sulfuric acid is needed as a catalyst. The general word equation for the reaction is: alcohol + organic acid → ester + water For example: methanol + butanoic acid → methyl butanoate + water
What Esters smell like
Different esters have different smells. These smells are often fruity.